Research centers
Being the result of a history that is more than half a century old, through 11 research centres, 410 researchers, 2 specialized libraries, 7 laboratories, FBK aims to results of excellence in science and technology with particular emphasis on interdisciplinary approaches and to the applicative dimension.
-
AUGMENTED INTELLIGENCE

Augmented Intelligence leverages artificial intelligence to enhance and improve human decision making, problem solving, and complex task execution through cooperative autonomous systems that amplify and extend human capabilities.
Read all -

The benefits of digital innovation come not without risks such as the loss of control over citizens' personal data, distortions of the democratic life of nations due to fake news and cyber-attacks that threaten critical infrastructures...
Read all -

Digital technologies are reshaping our society, and they are doing so with unprecedented intensity and speed; they are playing an ever greater role in our lives, deeply influencing culture, creativity, behavior, social life, leisure, education and work...
Read all -

The Digital Industry Center focuses its research on digital technologies for the manufacturing industry by creating applications for critical systems, adaptive and autonomous systems...
Read all -

The activities of the Center for Digital Health & Wellbeing mainly concern scientific research of excellence in the field of Computer Science and AI techniques and methodologies for health and healthcare...
Read all -

By combining knowledge of the epidemiology of infectious diseases and mathematical and computational modeling, the Health Emergencies Center develops quantitative epidemiology methods to strengthen epidemiological surveillance ...
Read all -

The Sustainable Energy Center is engaged in "decarbonization" efforts, which require greater flexibility in the energy system through the development of new and smarter gas and electricity networks and through the use of energy carriers...
Read all -
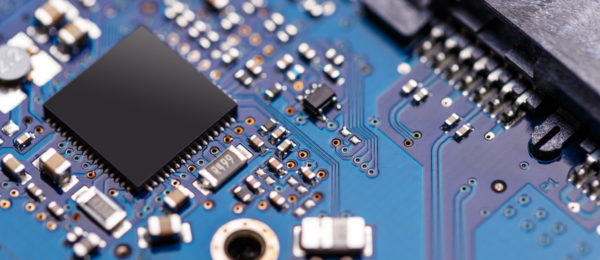
The SD Center focuses on highly integrated sensors and devices, products of excellence in research and industrial innovation, based on MEMS, CMOS, photonics and surface functionalization techniques and interfaces...
Read all -

The European Centre for Theoretical Studies in Nuclear Physics and Related Areas (ECT*) in Trento (Italy) provides a dedicated and structured combination of scientific activities for a large international scientific community...
Read all
-

Along with its research activity, The Research Institute for the Evaluation of Public Policies (FBK-IRVAPP) deals with the training of researchers, officials and public administrators...
Read all -

The Center for Religious Studies is an institute that deals with interdisciplinary research in the field of religion and is engaged in numerous research projects ...
Read all -

The Italian-German Historical Institute (ISIG) is a research center promoting study activities related to modern and contemporary history, with particular regard to the Italian and German areas...
Read all